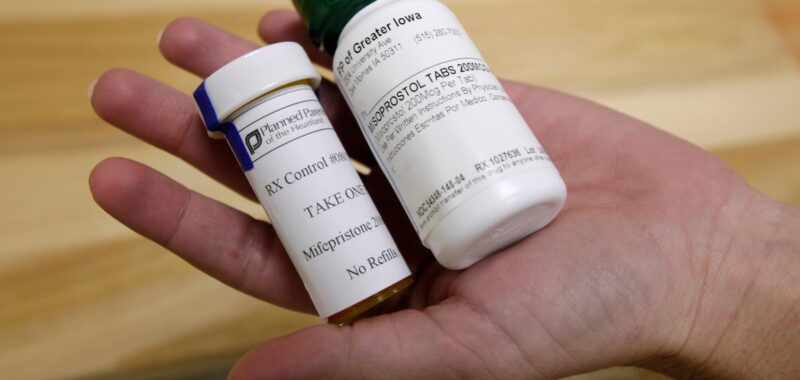
BATON ROUGE, La. — On Tuesday, Louisiana will become the first state in the U.S. to categorize two widely used abortion pills as “controlled dangerous substances.”
Opponents argue the classification could have catastrophic impacts in a state that already has a near-total abortion ban and one of the highest maternal mortality rates in the nation. Doctors fear the reclassification will cause delays in accessing the drugs — mifepristone and misoprostol — which together can be used to manage miscarriages, while misoprostol induces labor and treats severe bleeding after delivery. They also worry the practice of reclassifying the drugs might spread beyond Louisiana.
Proponents say the new law should help prevent coerced abortion, pointing to a Texas case in which a pregnant woman was given seven misoprostol pills by her husband without her knowledge; the baby survived. Over the past 15 years, news outlets have reported on similar cases — none in Louisiana — but the issue does not appear widespread.
Here’s what you should know about the new law.
Mifepristone and misoprostol can both be obtained through a prescription in Louisiana, but the state has reclassified the pills as “Schedule IV drugs,” putting them in the same category as the opioid tramadol and other substances that can be addictive.
Mifepristone is typically taken along with misoprostol and was approved by the U.S. Food and Drug Administration in 2000 after federal regulators deemed it safe and effective for ending pregnancies in the early weeks of gestation. The drug, which blocks the hormone progesterone, also primes the uterus to respond to the contraction-causing effect of misoprostol.
Last year in the U.S., nearly two-thirds of all abortions were medication abortions.
OB-GYNs say a tiny fraction of patients suffer “major” or “serious” adverse events after taking mifepristone. In June, the Supreme Court unanimously preserved access to the drug, throwing out a lawsuit from abortion opponents that argued the FDA overlooked serious safety problems when it made mifepristone easier to obtain.
Medical experts say it’s possible and safe to use misoprostol by itself to end a pregnancy, but it’s slightly less effective than the two-drug regimen. Besides being used in reproductive care, misoprostol is also used to prevent stomach ulcers in people who take certain pain medicines.
It depends.
Under current Louisiana law, physicians convicted of performing an illegal abortion, including one with pills, face up to 15 years in prison, $200,000 in fines and the loss of their medical license.
The new classification means that if someone knowingly possesses mifepristone or misoprostol without a valid prescription for any purpose, they could be fined up to $5,000 and sent to jail for one to five years.
The law carves out protections for pregnant women who obtain the drug without a prescription to take on their own.
Louisiana Attorney General Liz Murrill, a Republican who supports the current abortion ban and reclassification, said in September that the “intentional delivery of these drugs by organizations operating though the internet or other networks” is illegal and they will be prosecuted.
Doctors say the law could harm patients due to extra steps and more stringent storage requirements —especially in emergencies in which misoprostol is used to manage dangerous postpartum hemorrhages.
“As soon as the clock strikes midnight … this will be a reality almost immediately because we call for it in an emergency situation so frequently,” said Dr. Jane Martin, an OB-GYN at Ochsner Health in New Orleans whose hospital sees up to 5,000 births a year. It’s “administered at least once a day on labor and delivery,” often more frequently.
In hospitals like hers, misoprostol is usually stored in an OB-GYN unit in a “hemorrhage box” in the room, on the delivery table or in a nurse’s pocket, said Martin, who is active in the American College of Obstetricians and Gynecologists in Louisiana and stressed that she’s speaking for herself and not the hospital. With the new law, there will be more “red tape” to access the drugs — maybe down the hall in a locked container or potentially an in-house pharmacy at smaller hospitals.
Health experts said two alternative medications for hemorrhage have more side effects, can’t be used in patients with certain medical problems and need to be refrigerated.
Murrill countered that the law “does not limit a health care provider’s ability to use, prescribe, or fill these medications for legitimate health purposes nor does it impose restrictive burdens on access for emergency purposes.”
Dr. Kylie Cooper, a maternal-fetal specialist in Minnesota who is active with the American College of Obstetricians and Gynecologists, said that she’s concerned other states will adopt restrictions like Louisiana.
Up to 5% of obstetric patients will experience postpartum hemorrhages, which cause 11% of maternal deaths in the U.S., according to The Joint Commission, a nonprofit organization that sets standards and accredits health care organizations.
“Patients can lose a large amount of blood in a very, very short timeframe,” Cooper said. “So in many situations, seconds and minutes count.”
___
The Associated Press Health and Science Department receives support from the Howard Hughes Medical Institute’s Science and Educational Media Group and the Robert Wood Johnson Foundation. The AP is solely responsible for all content.